2026.02.09
Why Do Outcomes Differ Even with the “Same” NMN? —Understanding the Criteria for Selection Revealed Through Intranasal NMN—
This column is the final installment of a four-part series focusing on intranasal NMN.
In the previous articles, we have reviewed:
- Part 1: Why the Nose? —Thinking in Terms of Intranasal Delivery as a Brain-Focused Approach to NMN—
- Part 2: A Perspective on Insufficient Brain Energy —Can Reduced Concentration and Thinking Ability Be Explained as an Energy Shortage?—
- Part 3: How NMN Is Used Changes Its Meaning —Considering Oral, IV, and Intranasal Approaches Based on Purpose—
These perspectives have been gradually organized.
In this final article, we address a simple yet important question many readers ultimately face:
“The differences between methods are clear—but what criteria should I actually use to decide?”
A common misconception: Are there “different types” of NMN?
First, let us clarify a point that often causes confusion.
NMN (nicotinamide mononucleotide) is a single, chemically defined compound.
There is no such thing as “different NMN ingredients depending on the medical institution,” nor does a “special type” of NMN exist.
However, even when the chemical substance itself is identical, differences in formulation quality and handling may affect stability and condition at the time of use.
Why, then, do differences in perceived effects or evaluations arise?
Differences are not determined by the ingredient alone
In short, the reasons for such differences are not limited to the NMN compound itself.
NMN is considered to be sensitive to factors such as light, temperature, and humidity.
As a result,
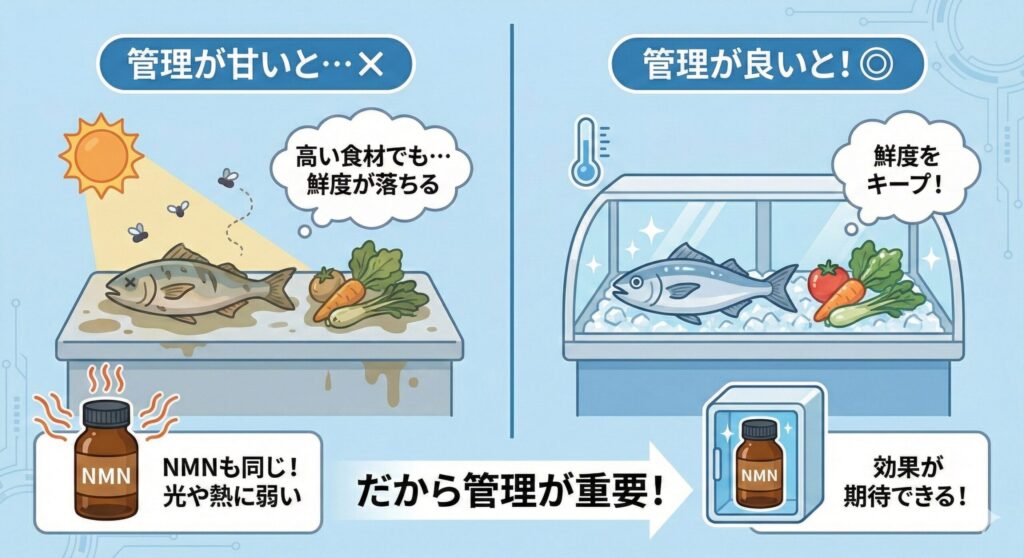
Why differences tend to be more noticeable with intranasal NMN
One reason differences are more noticeable with intranasal NMN is the process it undergoes from manufacturing until it actually enters the body.
This consideration is not irrelevant for oral or IV administration either.
However, intranasal administration has several characteristics:
- Relatively small administration volumes
- Dependence on delicate mucosal conditions
- Fewer intermediate steps such as digestion and metabolism
For these reasons, intranasal administration tends to reflect differences in management and handling more directly in user perception.
This does not mean that intranasal delivery is “unstable.”
Rather, it can be described as a method in which
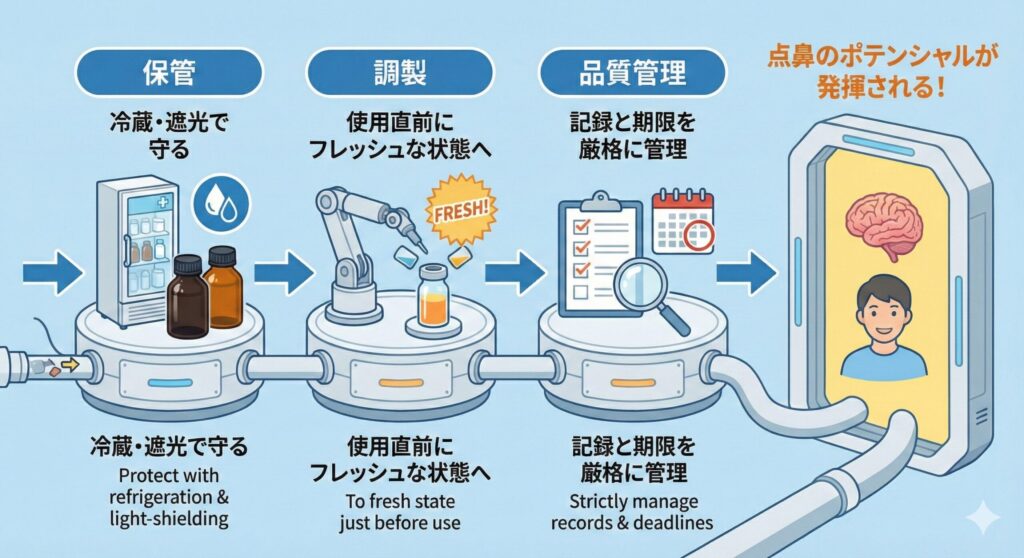
What is meant by “management and process”?
Although the terminology may sound technical, the concepts themselves are straightforward.
Specifically, they include:
- Storage: environments that maintain stability, such as light-protective containers and refrigeration
- Preparation: procedures that prevent degradation, such as dissolving immediately before use
- Quality control: management of expiration dates and storage records from production to use
The key point is not how “strict” these measures appear, but rather
Understanding purity correctly
When researching NMN, the term “purity” is often encountered.
Purity refers to the proportion of NMN within a given formulation.
Here again, caution is required.
- High purity does not automatically guarantee outcomes
- Lower purity does not necessarily indicate a problem
Higher purity generally means fewer impurities, but it does not by itself guarantee overall formulation quality.
What matters is whether storage conditions and handling after production are properly managed, in addition to purity.
An often-overlooked factor: the condition of the recipient
Another factor influencing evaluation is the physical condition and lifestyle of the individual receiving it.
Sleep, diet, and stress levels can all affect how something is perceived, a consideration not unique to NMN but common to many forms of health care.
Key points from this article
- NMN is chemically a single compound, but differences in stability and management can affect formulation quality
- Differences in perception and evaluation may arise from the combined effects of administration method, storage and preparation practices, and individual conditions
- Understanding these factors can support more informed and appropriate decision-making
Why differences in perception and evaluation occur
Looking back over this series, it becomes clear that differences in NMN experiences do not stem from a single factor.
- Each administration method has distinct characteristics
- Management practices such as storage and preparation vary
- Individual physical condition and lifestyle are not constant
When these factors overlap, differences in evaluation and perception are more likely to occur—even with the same NMN.
In closing
Information about NMN is abundant, and strong or definitive language is often encountered.
However, what this series has aimed to provide is not a single answer to “what should be chosen.”
Rather, it is about understanding the roles of different NMN administration methods and becoming able to make informed judgments based on that understanding.
We believe this represents the most realistic and satisfying conclusion.
If this series has helped clarify differences in approaches and ways of thinking, it has achieved its purpose.
This article is intended to introduce research background and perspectives only and does not indicate any specific effects or efficacy.
Please consult a physician before considering any treatment or use.

